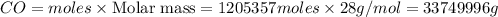The multistep smelting of ferric oxide to form elemental iron occurs at high temperatures in a blast furnace. In the first step, ferric oxide reacts with carbon monoxide to form Fe₃O.₄ This substance reacts with more carbon monoxide to formiron(II) oxide, which reacts with still more carbon monoxide to form molten iron. Carbon dioxide is also produced in each step.
(a) Write an overall balanced equation for the iron-smelting process.
(b) How many grams of carbon monoxide are required to form 45.0 metric tons of iron from ferric oxide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
The multistep smelting of ferric oxide to form elemental iron occurs at high temperatures in a blast...
Questions

Chemistry, 31.08.2019 14:00

Social Studies, 31.08.2019 14:00


Mathematics, 31.08.2019 14:00





History, 31.08.2019 14:10


Mathematics, 31.08.2019 14:10

Computers and Technology, 31.08.2019 14:10



Biology, 31.08.2019 14:10




History, 31.08.2019 14:10

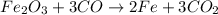
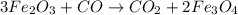
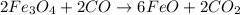
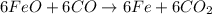
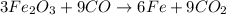
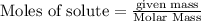
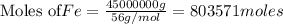
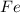 are produced from= 3 moles of
are produced from= 3 moles of 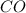
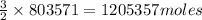 of
of