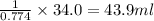Chemistry, 29.01.2021 16:30 elisakgarcia2007
The solubility in water of ionic compound X is measured and found to be 0.776g/mL at 15.°C. Calculate the volume of a saturated solution of X in water that would contain 34.0g of X at this temperature. Be sure your answer has the correct unit symbol and 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following best defines homeostasis? forming identical cells breaking down glucose maintaining stable internal conditions increasing an organism's temperature
Answers: 3

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3
You know the right answer?
The solubility in water of ionic compound X is measured and found to be 0.776g/mL at 15.°C. Calculat...
Questions

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Physics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Spanish, 16.09.2020 05:01

Spanish, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Spanish, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

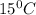 = 0.776 g/ml
= 0.776 g/ml