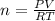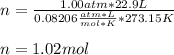Chemistry, 29.01.2021 14:00 aisatubrodie4626
50POINTS!
Using the ideal gas law (PV=nRT) solve for the missing. Variable. R= 0.08206atm*L/mol*k
If 22.9L of an ideal gas was collected at STP. How many moles of the gas were present?
A. 1.02 moles
B. 5.99 moles
C. 3.05 moles
D. 2.74 moles


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

You know the right answer?
50POINTS!
Using the ideal gas law (PV=nRT) solve for the missing. Variable. R= 0.08206atm*L/mol*k
Questions

Mathematics, 05.08.2020 02:01

Physics, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01

Biology, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01


Mathematics, 05.08.2020 02:01

Biology, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01


Mathematics, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01



Mathematics, 05.08.2020 02:01