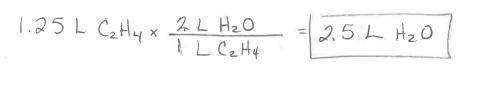Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Ethylene burns in oxygen to form carbon dioxide and water vapor:
C2H4(g) + 3 02(g) --> 2 CO2(g)...
Questions






Mathematics, 06.05.2020 07:27



Law, 06.05.2020 07:27

English, 06.05.2020 07:27

French, 06.05.2020 07:27





Computers and Technology, 06.05.2020 07:27

Mathematics, 06.05.2020 07:27


English, 06.05.2020 07:27

English, 06.05.2020 07:27