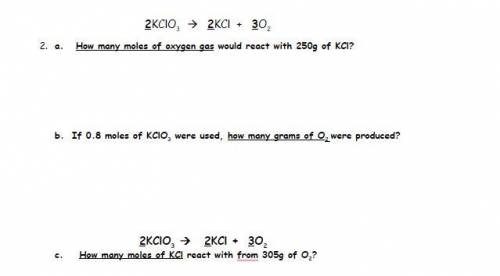
2KClO3 2KCl + 3O2
2 - A. How many moles of oxygen gas would react with 250g of KCl?
2 -...

Chemistry, 29.01.2021 03:30 shortty1111
2KClO3 2KCl + 3O2
2 - A. How many moles of oxygen gas would react with 250g of KCl?
2 - B. If 0.8 moles of KClO3 were used, how many grams of O2 were produced?
2- C. How many moles of KCl react with 305g of O2?
There are multiple questions for the chemical equation, so I've just combined them all into one.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
Questions

Mathematics, 03.02.2020 13:58



Biology, 03.02.2020 13:58



Mathematics, 03.02.2020 13:58


Social Studies, 03.02.2020 13:58

English, 03.02.2020 13:58

Mathematics, 03.02.2020 13:58

Mathematics, 03.02.2020 13:58

Health, 03.02.2020 13:58



Health, 03.02.2020 13:58



History, 03.02.2020 13:58

Mathematics, 03.02.2020 13:58



