Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1

Chemistry, 23.06.2019 13:30
How many ammonium ions and how many sulfate ions are present in a 0.270 mol sample of ?
Answers: 1

Chemistry, 23.06.2019 17:30
Which of the following elements would you expect to have the highest ionization energy value, and why? a. chlorine (cl), because it has a low effective nuclear charge and large radius b. fluorine (f), because it has a large radius and naturally forms a negative ion c. lithium (li), because it has a small radius and naturally forms a positive ion d. neon (ne), because it has a high effective nuclear charge and small radius
Answers: 2
You know the right answer?
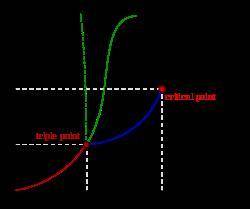
Nitrogen can exist as a solid, a liquid, or a gas. Which of the following lists the
phases of nitro...
Questions



Mathematics, 21.02.2021 03:20




Mathematics, 21.02.2021 03:20



Geography, 21.02.2021 03:20

Business, 21.02.2021 03:20

History, 21.02.2021 03:20

Mathematics, 21.02.2021 03:20


Mathematics, 21.02.2021 03:20

History, 21.02.2021 03:20

Mathematics, 21.02.2021 03:20

Advanced Placement (AP), 21.02.2021 03:20