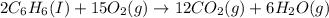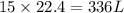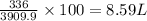Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
How many liters of oxygen gas (O2) are needed to produce 100 kJ of energy at STP? 2C6H6(I) + 1502(g)...
Questions

Mathematics, 12.01.2020 07:31

Mathematics, 12.01.2020 07:31



Mathematics, 12.01.2020 07:31

English, 12.01.2020 07:31

Health, 12.01.2020 07:31

Mathematics, 12.01.2020 07:31




Mathematics, 12.01.2020 07:31



History, 12.01.2020 07:31


Mathematics, 12.01.2020 07:31

Mathematics, 12.01.2020 07:31


Mathematics, 12.01.2020 07:31

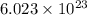 of particles.
of particles.