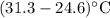Chemistry, 27.01.2021 20:10 myhomeacc32
In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of 1M HCl are mixed. The HCl had an initial temperature of 44.6 C and the water was originally at 24.6 C. After the reaction, the temperature of both substances is 31.3 C.
Required:
a. Was the reaction exothermic or endothermic? Explain.
b. Calculate how much heat the water lost or gained.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 23.06.2019 16:30
Answer immediately when two or more atoms of the same element are in a chemical bond, the substance is called a(n) a. compound b. molecule c. nucleus
Answers: 1

Chemistry, 23.06.2019 19:30
What are the two most influential greenhouse gases in maintaining the earth's climate? a) carbon dioxide & silicon b) water vapor & carbon dioxide c) silicon & oxygen d) nitrogen & water vapor
Answers: 2
You know the right answer?
In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of 1M HCl are mixed. The HCl had an initial...
Questions

English, 05.10.2019 10:01


History, 05.10.2019 10:01


Mathematics, 05.10.2019 10:01

Mathematics, 05.10.2019 10:01

Chemistry, 05.10.2019 10:01

History, 05.10.2019 10:01






Computers and Technology, 05.10.2019 10:01





Engineering, 05.10.2019 10:01


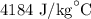
 = Change in temperature of water =
= Change in temperature of water =