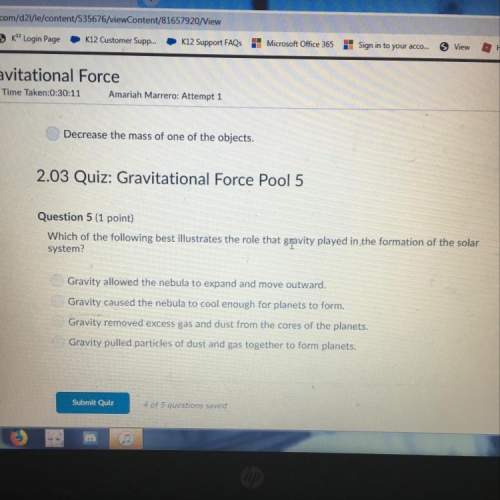
Chemistry, 26.01.2021 22:10 Satoetoe24
Calculate the number of grams of solid aluminium chloride that will form when a mixture containing 0.150 g of aluminum powder and 1.00 g of chlorine gas is allowed to react.
2Al (s) + 3Cl2 (g) ---> 2AlCl3 (s)
a. 741 g AlCl3
b. 471 g AlCl3
c. 0.741 g AlCl3
d. 246 g AlCl3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1

Chemistry, 23.06.2019 14:30
Most points for whoever anwers i need your which is not an isotope of carbon? a. carbon-6 b. carbon-12 c. carbon-13 d. carbon-14
Answers: 2
You know the right answer?
Calculate the number of grams of solid aluminium chloride that will form when a mixture containing 0...
Questions





Chemistry, 09.11.2019 08:31


Mathematics, 09.11.2019 08:31



Social Studies, 09.11.2019 08:31

Biology, 09.11.2019 08:31