The kp for the reaction below is 1.49 × 108 at 100.0°c:
co(g) + cl2(g) → cocl2(g)
...

The kp for the reaction below is 1.49 × 108 at 100.0°c:
co(g) + cl2(g) → cocl2(g)
in an equilibrium mixture of the three gases, pco = pcl2 = 2.22 × 10-4 atm. the partial pressure of the product, phosgene (cocl2), is atm.
a) 7.34
b) 3.02 × 10^15
c) 3.31 × 10^-16
d) 3.31 × 10^4
e) 6.67 × 10^11

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3

Chemistry, 23.06.2019 15:40
Twenty-seven milliliters of an acid with an unknown concentration are titrated with a base that has a concentration of 0.55 m. the indicator changed color when 12.5 milliliters of base were added. what is the concentration of the unknown acid?
Answers: 2

Chemistry, 23.06.2019 16:10
What type of reaction is shown below? check all that apply. agno3(aq) + nacl(aq) → nano3(aq) + agcl(s) i synthesis decomposition combustion i single replacement double replacement done
Answers: 2

You know the right answer?
Questions

Mathematics, 23.11.2021 01:00

Mathematics, 23.11.2021 01:00


Mathematics, 23.11.2021 01:00




Chemistry, 23.11.2021 01:00

Chemistry, 23.11.2021 01:00

Mathematics, 23.11.2021 01:00




English, 23.11.2021 01:00

Mathematics, 23.11.2021 01:00


Social Studies, 23.11.2021 01:00

Mathematics, 23.11.2021 01:00


Mathematics, 23.11.2021 01:00

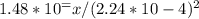 . So, the correct answer is A) 7.34.
. So, the correct answer is A) 7.34.

