
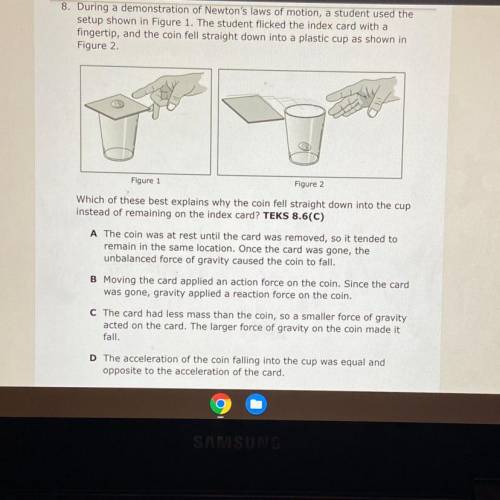
During a demonstration of Newton's laws of motion, a student used the
setup shown in Figure 1. The student flicked the index card with a
fingertip, and the coin fell straight down into a plastic cup as shown in
Figure 2.
Figure 1
Figure 2
Which of these best explains why the coin fell straight down into the cup
instead of remaining on the index card? TEKS 8.6(C)
A The coin was at rest until the card was removed, so it tended to
remain in the same location. Once the card was gone, the
unbalanced force of gravity caused the coin to fall.
B Moving the card applied an action force on the coin. Since the card
was gone, gravity applied a reaction force on the coin.
C The card had less mass than the coin, so a smaller force of gravity
acted on the card. The larger force of gravity on the coin made it
fall.
D The acceleration of the coin falling into the cup was equal and
opposite to the acceleration of the card.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
During a demonstration of Newton's laws of motion, a student used the
setup shown in Figure 1. The...
Questions

History, 19.03.2021 23:50

Health, 19.03.2021 23:50



Biology, 19.03.2021 23:50


Mathematics, 19.03.2021 23:50

Computers and Technology, 19.03.2021 23:50

Mathematics, 19.03.2021 23:50







Mathematics, 19.03.2021 23:50

Mathematics, 19.03.2021 23:50


Mathematics, 19.03.2021 23:50




