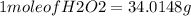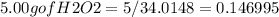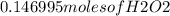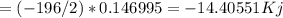Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l)...

Chemistry, 19.01.2020 09:31 calvinclifton
Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l) 2h2o(l) + o2(g) enthalpy=-196kj
calculate the value of q when 5.00g of h20(l) decomposes at constant pressure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1


Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1
You know the right answer?
Questions


Mathematics, 16.07.2019 23:30

Biology, 16.07.2019 23:30

Biology, 16.07.2019 23:30

Mathematics, 16.07.2019 23:30

Computers and Technology, 16.07.2019 23:30

Health, 16.07.2019 23:30


Biology, 16.07.2019 23:30

Biology, 16.07.2019 23:30



Biology, 16.07.2019 23:30



Social Studies, 16.07.2019 23:30



Mathematics, 16.07.2019 23:30