Chemistry, 26.01.2021 16:50 nessabear9472
In the formation of acid rain, sulfur dioxide reacts with
oxygen and water vapor in the air to form sulfuric acid. If
6.4 mol of SO2 react with excess oxygen and water, how
many moles of H2SO4 are produced?
2502 + O2 + 2H20 → 2H2SO4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
In the formation of acid rain, sulfur dioxide reacts with
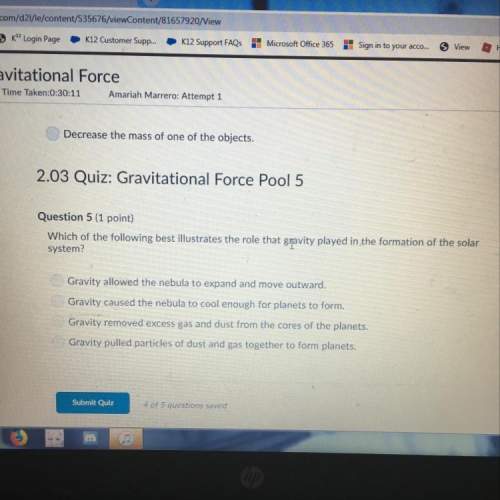
oxygen and water vapor in the air to form...
Questions


Mathematics, 17.09.2021 17:10


History, 17.09.2021 17:10

Engineering, 17.09.2021 17:10

History, 17.09.2021 17:10




Mathematics, 17.09.2021 17:10

Mathematics, 17.09.2021 17:10






English, 17.09.2021 17:10

SAT, 17.09.2021 17:10

Mathematics, 17.09.2021 17:10