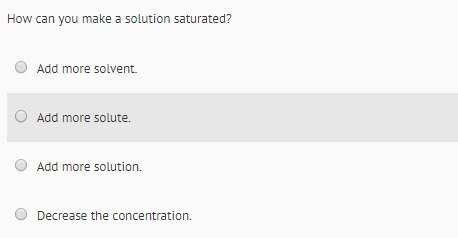
Which of the following is TRUE?
-insoluble ionic compounds are strong electrolytes.
-the hig...

Chemistry, 26.01.2021 16:00 NoahJerowski67
Which of the following is TRUE?
-insoluble ionic compounds are strong electrolytes.
-the higher the temperature, the higher the solubility of a gas in a liquid.
-the more solute particles in solution, the greater the effect on freezing point and boiling point.
-strong acids that dissociate completely into ions are non-electrolytes.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Questions

Mathematics, 03.02.2021 21:30



Chemistry, 03.02.2021 21:30





Mathematics, 03.02.2021 21:30


Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30



Mathematics, 03.02.2021 21:30



History, 03.02.2021 21:30

History, 03.02.2021 21:30