Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1

Chemistry, 23.06.2019 15:30
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
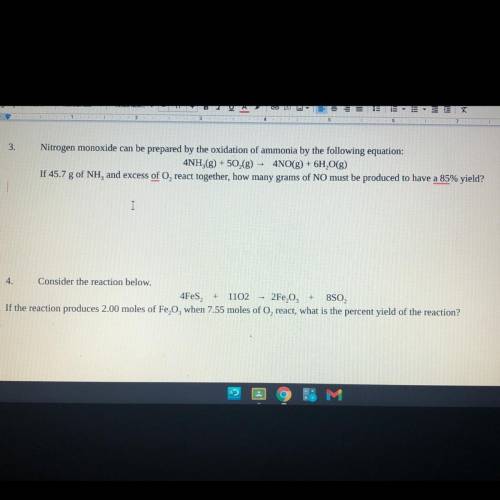
Consider the reaction below.
4Fes2 + 11O2 —> 2Fe2O3 + 8SO2
If the reaction produces 2.00 m...
If the reaction produces 2.00 m...
Questions

English, 13.11.2019 00:31


Physics, 13.11.2019 00:31








Mathematics, 13.11.2019 01:31

Mathematics, 13.11.2019 01:31

Health, 13.11.2019 01:31




Arts, 13.11.2019 01:31

English, 13.11.2019 01:31


History, 13.11.2019 01:31