
Chemistry, 25.01.2021 21:40 911wgarcia
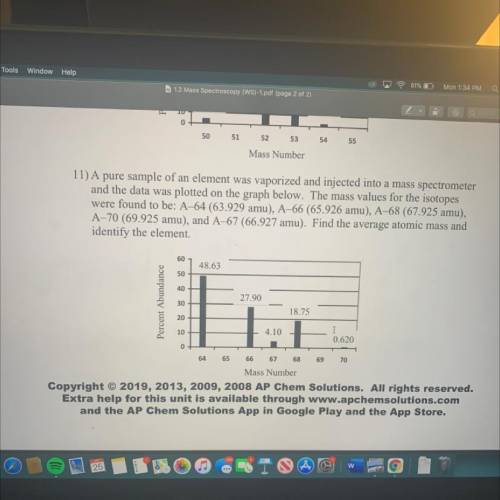
11) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data was plotted on the graph below. The mass values for the isotopes
were found to be: A–64 (63.929 amu), A–66 (65.926 amu), A-68 (67.925 amu),
A-70 (69.925 amu), and A-67 (66.927 amu). Find the average atomic mass and
identify the element.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
11) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data wa...
Questions

Chemistry, 26.09.2019 11:50

Mathematics, 26.09.2019 11:50


English, 26.09.2019 11:50

Mathematics, 26.09.2019 11:50


History, 26.09.2019 12:00




Mathematics, 26.09.2019 12:00

Mathematics, 26.09.2019 12:00




History, 26.09.2019 12:00



Biology, 26.09.2019 12:00



