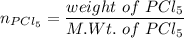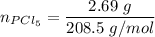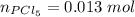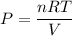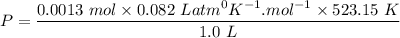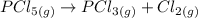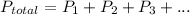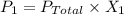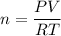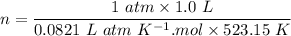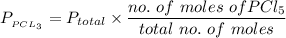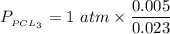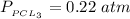Chemistry, 25.01.2021 21:10 Queenbee2304
A sample of PCl5 weighting 2.69 gram was placed in 1.00 Litter container and completely vaporized at 250C. The pressure observed at that temperature was 1.00 atm. The possibility exists that some of the PCl5 dissociated according to PCl5 (g) ! PCl3 (g) Cl2 (g) . What must be the partial pressures of PCl5 PCl3 and Cl2 under these experimental conditions

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 13:00
The gram molecular mass or co2 is the same as the gram molecular mass of
Answers: 2

Chemistry, 23.06.2019 15:30
Which term defines a type of oxygen that forms a protective layer miles above the earth a. fossil fuel b. smog c. pollution d. ozone
Answers: 2
You know the right answer?
A sample of PCl5 weighting 2.69 gram was placed in 1.00 Litter container and completely vaporized at...
Questions

French, 29.11.2021 01:20

Physics, 29.11.2021 01:20

Mathematics, 29.11.2021 01:20

History, 29.11.2021 01:20

Business, 29.11.2021 01:20

Mathematics, 29.11.2021 01:20


Mathematics, 29.11.2021 01:30

History, 29.11.2021 01:30


Mathematics, 29.11.2021 01:30

Mathematics, 29.11.2021 01:30


Mathematics, 29.11.2021 01:30


Mathematics, 29.11.2021 01:30



Mathematics, 29.11.2021 01:30