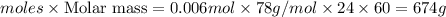The CO2 that builds up in the air of a submerged submarine can be removed by reacting it with sodium peroxide. 2 Na2O2 (s) + 2 CO2 (g) → 2 Na2CO3 (s) + O2 (g) If a sailor exhales 150.0 mL of CO2 per minute at 20oC and 1.02 atm, how much sodium peroxide is needed per sailor in a 24 hr period?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
The CO2 that builds up in the air of a submerged submarine can be removed by reacting it with sodium...
Questions

Mathematics, 29.07.2019 23:40




Mathematics, 29.07.2019 23:40

English, 29.07.2019 23:40

Mathematics, 29.07.2019 23:40


Mathematics, 29.07.2019 23:40

Health, 29.07.2019 23:40


Mathematics, 29.07.2019 23:40



Mathematics, 29.07.2019 23:40


Physics, 29.07.2019 23:40

History, 29.07.2019 23:40


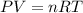
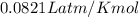
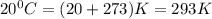
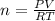
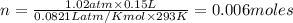
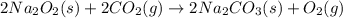
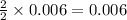 moles of sodium peroxide
moles of sodium peroxide