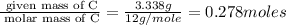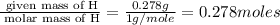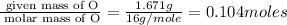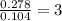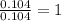Chemistry, 25.01.2021 20:30 batpool3375
Methyl salicylate is a common active ingredient in liniments such as ben-gay. It is also known as oil of wintergreen. It is made up of carbon, hydrogen, and oxygen atoms. When a sample of methyl salicylate weighing 5.287 g is burned in excess oxygen, 12.24 g of carbon dioxide and 2.505 g of water are formed. What is the simplist formula for oil of wintergreen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
Methyl salicylate is a common active ingredient in liniments such as ben-gay. It is also known as oi...
Questions

English, 19.05.2021 18:00



Advanced Placement (AP), 19.05.2021 18:00



Mathematics, 19.05.2021 18:00

English, 19.05.2021 18:10

English, 19.05.2021 18:10

Social Studies, 19.05.2021 18:10

Mathematics, 19.05.2021 18:10

Business, 19.05.2021 18:10

Arts, 19.05.2021 18:10



Chemistry, 19.05.2021 18:10




Mathematics, 19.05.2021 18:10

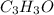 .
.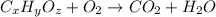
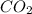 = 12.24 g
= 12.24 g
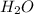 = 2.505 g
= 2.505 g
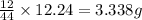 of carbon will be contained.
of carbon will be contained.
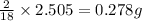 of hydrogen will be contained.
of hydrogen will be contained.