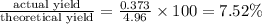Chemistry, 25.01.2021 18:00 MikeCrotch19251
2H2 (1) + O2(g) → 2H20 (g)
1. Find the limiting reactant if you start with 30.0 grams of hydrogen and 5.29 grams of oxygen.
2. The actual yield for H2O in the above reaction is 6.72 g, Determine the percent yield for the reaction
when 9.93 grams of hydrogen and excess oxygen react?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
2H2 (1) + O2(g) → 2H20 (g)
1. Find the limiting reactant if you start with 30.0 grams of hydrogen a...
Questions

Biology, 22.03.2021 18:10

Mathematics, 22.03.2021 18:10

Mathematics, 22.03.2021 18:10

English, 22.03.2021 18:10



Mathematics, 22.03.2021 18:20

History, 22.03.2021 18:20

Mathematics, 22.03.2021 18:20


English, 22.03.2021 18:20

Mathematics, 22.03.2021 18:20

English, 22.03.2021 18:20

Arts, 22.03.2021 18:20

Mathematics, 22.03.2021 18:20

Mathematics, 22.03.2021 18:20




Chemistry, 22.03.2021 18:20

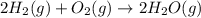
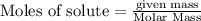
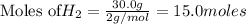
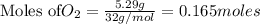
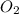 require = 2 moles of
require = 2 moles of 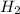
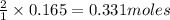 of
of 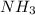
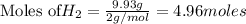
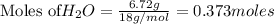
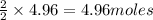 of
of