
Chemistry, 25.01.2021 04:10 09daishagreen
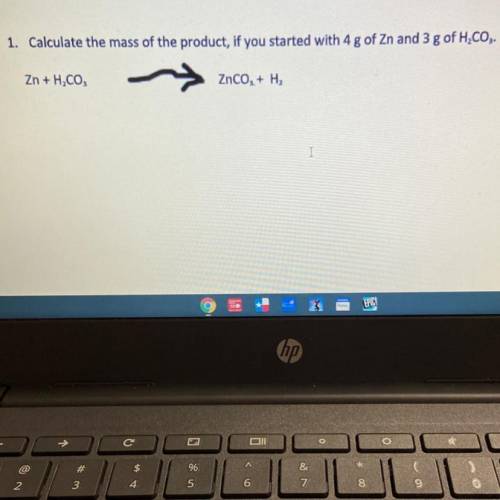
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H, CO,
ZnCO, + H2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1


Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H, CO,
Questions


English, 26.06.2019 22:40





Mathematics, 26.06.2019 22:40

English, 26.06.2019 22:40







Mathematics, 26.06.2019 22:40







