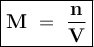A chemist prepares a solution of potassium iodide (KI) by measuring out 88. umol of potassium iodide into a 200. mL volumetric flask and filling the flask to
the mark with water.
Calculate the concentration in mol/L of the chemist's potassium iodide solution. Round your answer to 2 significant digits.
Please Help me!


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
A chemist prepares a solution of potassium iodide (KI) by measuring out 88. umol of potassium iodide...
Questions


Mathematics, 04.04.2021 18:00

Mathematics, 04.04.2021 18:00



Mathematics, 04.04.2021 18:00


History, 04.04.2021 18:00


Social Studies, 04.04.2021 18:00

Mathematics, 04.04.2021 18:00

Mathematics, 04.04.2021 18:00


Mathematics, 04.04.2021 18:00



English, 04.04.2021 18:00

History, 04.04.2021 18:00

English, 04.04.2021 18:00

Social Studies, 04.04.2021 18:00