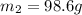Chemistry, 04.02.2020 11:54 tamikeen2243
To a sample of water at 23.4oc in a constant pressure calorimeter of negligible heat capacity is added a 12.1 g piece of aluminium whose temperature is 81.7oc. if the final temperature of water is 24.9 oc, calculate the mass of the water in the calorimeter. ans: 98.6g
-i know that the specific heat of aluminum is 0.900 j/g ‡ ác
- _t al is 24.9ác _ 81.7ác = _56.8ác
- _twater and _tcalorimeter are both 24.9ác _ 23.4ác = 1.5ác.
-the specific heat of water is 4.184 j/g ‡ ác.
but i tried using m=q/s_t. i'm really stuck, can anyone me?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
To a sample of water at 23.4oc in a constant pressure calorimeter of negligible heat capacity is add...
Questions

Biology, 20.09.2020 16:01

English, 20.09.2020 16:01

Computers and Technology, 20.09.2020 16:01

Social Studies, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

English, 20.09.2020 16:01

Health, 20.09.2020 16:01


English, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01





History, 20.09.2020 16:01

English, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

Biology, 20.09.2020 16:01

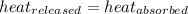
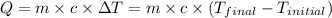
![-m_1\times c_1\times (T_{final}-T_1)=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0500/2981/27fc4.png) .................(1)
.................(1)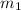 = mass of aluminium = 12.1 g
= mass of aluminium = 12.1 g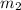 = mass of water = ?
= mass of water = ?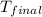 = final temperature =
= final temperature = 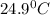
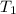 = temperature of aluminium =
= temperature of aluminium = 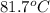
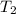 = temperature of water =
= temperature of water = 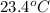
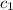 = specific heat of aluminium =
= specific heat of aluminium = 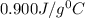
 = specific heat of water=
= specific heat of water= 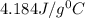
![-12.1\times 0.900\times (24.9-81.7)=-[m_2\times 4.184\times (24.9-23.4)]](/tpl/images/0500/2981/9ba9b.png)