
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) with 0.210 m koh(aq).
(a) before addition of any koh
(b) after addition of 25.0 ml of koh
(c) after addition of 35.0 ml of koh
(d) after addition of 50.0 ml of koh
(e) after addition of 60.0 ml of koh

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) wit...
Questions



Mathematics, 27.07.2019 04:20








History, 27.07.2019 04:20

Mathematics, 27.07.2019 04:20

Mathematics, 27.07.2019 04:20

Mathematics, 27.07.2019 04:20




Computers and Technology, 27.07.2019 04:20


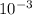
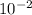
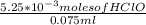 = 0.070M
= 0.070M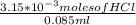 = 0.037M
= 0.037M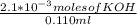 = 0.020M
= 0.020M


