
Chemistry, 23.01.2021 06:50 johnnyhalusewa
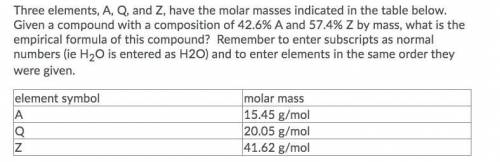
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound with a composition of 42.6% A and 57.4% Z by mass, what is the empirical formula of this compound? Remember to enter subscripts as normal numbers (ie H2O is entered as H2O) and to enter elements in the same order they were given.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3

Chemistry, 23.06.2019 17:30
Alithium atom has three protons, three neutrons, and three electrons. what is the overall charge on this atom?
Answers: 1
You know the right answer?
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound wi...
Questions



English, 01.09.2019 18:30

History, 01.09.2019 18:30

Mathematics, 01.09.2019 18:30

Spanish, 01.09.2019 18:30


Biology, 01.09.2019 18:30


Social Studies, 01.09.2019 18:30

Biology, 01.09.2019 18:30




History, 01.09.2019 18:30


Mathematics, 01.09.2019 18:30


Mathematics, 01.09.2019 18:30

Computers and Technology, 01.09.2019 18:30



