
Chemistry, 22.01.2021 21:10 npellot123
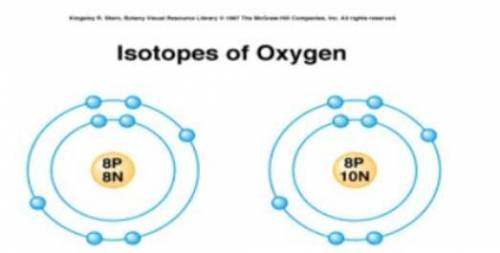
Students were shown models of two atoms and asked to make a list of similarities and differences between the models.
Which of the statements about the atomic models shown is correct?
A) Both models represent atoms with the same atomic number.
B) Both models represent atoms with the same atomic mass.
C) The model on the left is an ion and the model on the right is an isotope.
D) The model on the left is an isotope and the model on the right is an ion.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
Students were shown models of two atoms and asked to make a list of similarities and differences bet...
Questions

Computers and Technology, 03.05.2021 09:10


Chemistry, 03.05.2021 09:10

Arts, 03.05.2021 09:10

Mathematics, 03.05.2021 09:10

Mathematics, 03.05.2021 09:10



Social Studies, 03.05.2021 09:10

Chemistry, 03.05.2021 09:10

Computers and Technology, 03.05.2021 09:10

Social Studies, 03.05.2021 09:10


Physics, 03.05.2021 09:10


Mathematics, 03.05.2021 09:10


Computers and Technology, 03.05.2021 09:10

Computers and Technology, 03.05.2021 09:10



