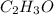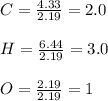Chemistry, 22.01.2021 19:00 jeffffffff
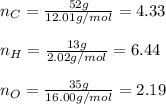
A percent composition analysis yields 52% carbon (C), 13% hydrogen (H), and 35% oxygen (O). What is the empirical formula for the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1
You know the right answer?
A percent composition analysis yields 52% carbon (C), 13% hydrogen (H), and 35% oxygen (O). What is...
Questions


History, 11.11.2020 14:40

Mathematics, 11.11.2020 14:40

Biology, 11.11.2020 14:40

English, 11.11.2020 14:50

Spanish, 11.11.2020 14:50

Mathematics, 11.11.2020 14:50


Mathematics, 11.11.2020 14:50


Social Studies, 11.11.2020 14:50

Mathematics, 11.11.2020 14:50

Chemistry, 11.11.2020 14:50




Mathematics, 11.11.2020 14:50

Mathematics, 11.11.2020 14:50


History, 11.11.2020 14:50