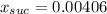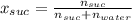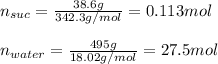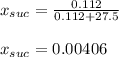Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


You know the right answer?
A solution is prepared by dissolving 38.6 g sucrose (C12H22O11) in 495 g of water. Determine the mol...
Questions


History, 24.08.2019 19:10

Health, 24.08.2019 19:10


Chemistry, 24.08.2019 19:10



Health, 24.08.2019 19:10


Mathematics, 24.08.2019 19:10




Computers and Technology, 24.08.2019 19:10



Mathematics, 24.08.2019 19:10


History, 24.08.2019 19:10