

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
Acompound is decomposed in the laboratory and produces 1.40 g n and 0.20g h what is the empirical fo...
Questions



Social Studies, 12.08.2019 18:10
















Computers and Technology, 12.08.2019 18:10

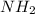 .
.


