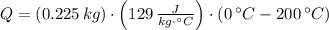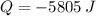Chemistry, 22.01.2021 18:30 jarednash015
What is the total amount of heat needed to change 2250 g of silver at 200.0°C to 0.0°C? The specific heat of silver is 0.129 J/g∙°C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2

Chemistry, 23.06.2019 12:50
Acertain reaction has a activation energy of 54.0 kj/mol. as the temperature is increased from 22c to a higher temperature, the rate constant increases by a factor of 7.00. calculate the higher temperature. c (report only numerical answer)
Answers: 3

Chemistry, 23.06.2019 15:00
For part 1, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least copper(ii) nitrate to the well with the most copper(ii) nitrate. which wells had the most distinct precipitate? for part 2, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least iron(ii) sulfate to the well with the most iron(ii) sulfate. which wells had the most distinct precipitate? for part 3, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least iron(iii) nitrate to the well with the most iron(iii) nitrate. which wells had the most distinct precipitate?
Answers: 3
You know the right answer?
What is the total amount of heat needed to change 2250 g of silver at 200.0°C to 0.0°C? The specific...
Questions

History, 21.09.2020 21:01










Mathematics, 21.09.2020 21:01

Mathematics, 21.09.2020 21:01





History, 21.09.2020 21:01

English, 21.09.2020 21:01


 ), measured in joules, is determined by the following formula:
), measured in joules, is determined by the following formula: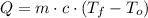 (1)
(1)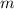 - Mass, measured in kilograms.
- Mass, measured in kilograms. - Specific heat of silver, measured in joules per grams-degrees Celsius.
- Specific heat of silver, measured in joules per grams-degrees Celsius.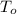 ,
, 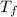 - Initial and final temperatures, measured in degrees Celsius.
- Initial and final temperatures, measured in degrees Celsius. 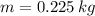 ,
, 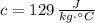 ,
, 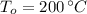 and
and 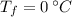 , then the heat received by the sample of silver is:
, then the heat received by the sample of silver is: