
Chemistry, 22.01.2021 16:00 robbiegfarmer
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound with a composition of 42.9% A, 18.6% Q and 38.5% Z by mass, what is the empirical formula of this compound?
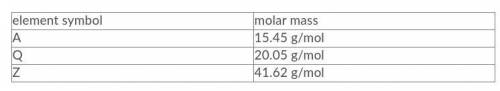

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound wi...
Questions



Mathematics, 27.05.2020 05:00



Mathematics, 27.05.2020 05:00




Mathematics, 27.05.2020 05:00





Biology, 27.05.2020 05:00

Computers and Technology, 27.05.2020 05:00

Business, 27.05.2020 05:00





