APPLY YOUR KNOWLEDGE
Graphite and diamond are different forms of the element carbon.
Graphite...

Chemistry, 22.01.2021 14:00 anacecilianr2325
APPLY YOUR KNOWLEDGE
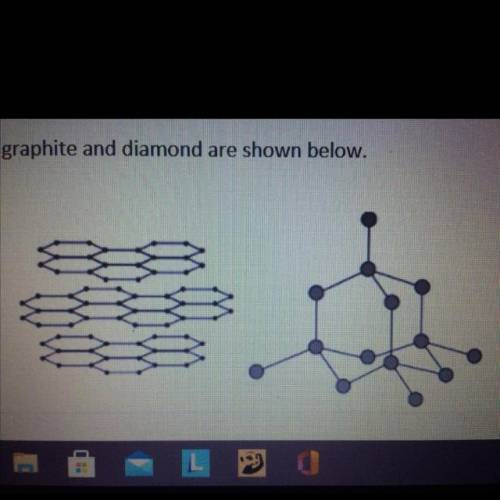
Graphite and diamond are different forms of the element carbon.
Graphite and diamond have different properties.
The structures of graphite and diamond are shown below.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1


Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
Questions




Mathematics, 18.07.2021 03:30


Mathematics, 18.07.2021 03:30

Mathematics, 18.07.2021 03:30

Computers and Technology, 18.07.2021 03:30

History, 18.07.2021 03:30


Physics, 18.07.2021 03:30



Mathematics, 18.07.2021 03:30

Mathematics, 18.07.2021 03:30

English, 18.07.2021 03:30



Computers and Technology, 18.07.2021 03:30



