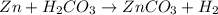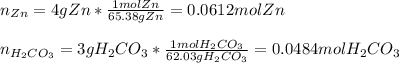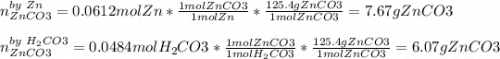Chemistry, 22.01.2021 08:30 davidtemple
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H2CO3
ZnCO2 + H2 * I’ll cash app for the right answer plus explanation”


Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3
You know the right answer?
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H2CO3
Questions


Mathematics, 21.04.2021 17:00




History, 21.04.2021 17:00

English, 21.04.2021 17:00

Mathematics, 21.04.2021 17:00




Mathematics, 21.04.2021 17:00

Mathematics, 21.04.2021 17:00

Mathematics, 21.04.2021 17:00

Social Studies, 21.04.2021 17:00


Mathematics, 21.04.2021 17:00


Physics, 21.04.2021 17:00

Mathematics, 21.04.2021 17:00