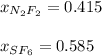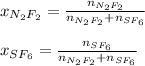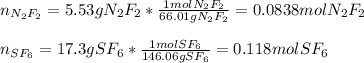Chemistry, 21.01.2021 23:00 darknessmidnight207
A 8.00 L tank at 26.9 C is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
A 8.00 L tank at 26.9 C is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexa...
Questions

Mathematics, 22.12.2020 02:30


Social Studies, 22.12.2020 02:30

English, 22.12.2020 02:30


Mathematics, 22.12.2020 02:30

Mathematics, 22.12.2020 02:30

Mathematics, 22.12.2020 02:30

Mathematics, 22.12.2020 02:30



Mathematics, 22.12.2020 02:30

Physics, 22.12.2020 02:30




Mathematics, 22.12.2020 02:30

Mathematics, 22.12.2020 02:40

Mathematics, 22.12.2020 02:40