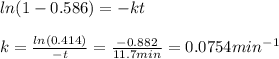Chemistry, 21.01.2021 22:20 DerekMoncoal
In a first-order decomposition reaction. 58.6% of a compound decomposes in 11.7 min. How long (in min) does it take for 80.2% of the compound to decompose

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
In a first-order decomposition reaction. 58.6% of a compound decomposes in 11.7 min. How long (in mi...
Questions

English, 23.03.2020 02:18

Mathematics, 23.03.2020 02:18

Mathematics, 23.03.2020 02:19

Mathematics, 23.03.2020 02:19

Mathematics, 23.03.2020 02:19

Mathematics, 23.03.2020 02:19

Mathematics, 23.03.2020 02:19

Mathematics, 23.03.2020 02:19

Mathematics, 23.03.2020 02:19

Mathematics, 23.03.2020 02:19

Chemistry, 23.03.2020 02:19





Mathematics, 23.03.2020 02:20

History, 23.03.2020 02:21


Mathematics, 23.03.2020 02:21

Mathematics, 23.03.2020 02:21

![\frac{[A]}{[A]_0}=exp(-kt)](/tpl/images/1053/6847/9e1e3.png)