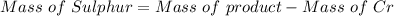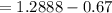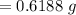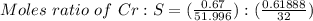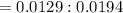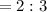Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
A 0.67 gram sample of chromium is reacted with sulfur. The resulting chromium sulfide has a mass of...
Questions



Mathematics, 11.03.2021 17:40

French, 11.03.2021 17:40




Health, 11.03.2021 17:40






Mathematics, 11.03.2021 17:40


Chemistry, 11.03.2021 17:40


Mathematics, 11.03.2021 17:40

English, 11.03.2021 17:40

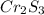 ".
".