Chemistry, 21.01.2021 22:10 itssamuelu
The following data were collected for the reaction between hydrogen and nitric oxide at 700°C: 2H2(g) + 2NO(g) -+ 2H20(g) + N2(g) Experiment [H2]/M [NO]/M Initial rate/M. s-1 1 0.010 0.025 2.4 X 10-6 2 0.0050 0.025 1.2 X 10-6 3 0.010 0.0125 0.60 X 10-6 (a) What is the rate law for the reaction? (b) Calculate the rate constant for the reaction. (c) Suggest a plausible reaction mechanism that is consistent with the rate

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1


Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
The following data were collected for the reaction between hydrogen and nitric oxide at 700°C: 2H2(g...
Questions


Mathematics, 22.02.2021 22:30

Chemistry, 22.02.2021 22:30



Mathematics, 22.02.2021 22:30


English, 22.02.2021 22:30







Chemistry, 22.02.2021 22:30

Mathematics, 22.02.2021 22:30



Mathematics, 22.02.2021 22:30

![rate = k[H_2]_x [NO]_y](/tpl/images/1053/5726/53919.png)
![rate = k[H_2][NO]_2](/tpl/images/1053/5726/46bd0.png)
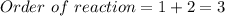
![k = \dfrac{rate}{ [H_2] [NO]^2}](/tpl/images/1053/5726/8cad0.png)