Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2


Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
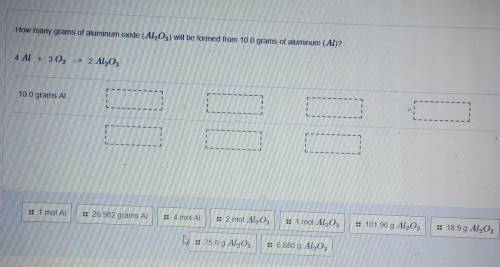
How many grams of aluminum oxide (Al2O3) will be formed from 10.0 grams of aluminum (Al)? 4 AL + 3 0...
Questions


Mathematics, 14.07.2020 18:01





History, 14.07.2020 18:01


Biology, 14.07.2020 18:01


Mathematics, 14.07.2020 18:01






Social Studies, 14.07.2020 18:01


Mathematics, 14.07.2020 18:01

Mathematics, 14.07.2020 18:01