
Chemistry, 21.01.2021 15:40 cxttiemsp021
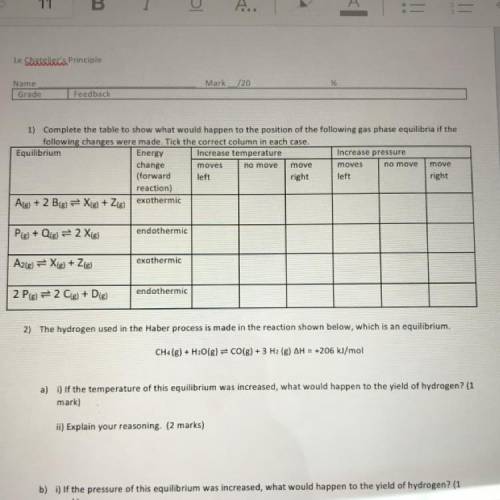
1) Complete the table to show what would happen to the position of the following gas phase equilibria if the
following changes were made. Tick the correct column in each case.
Equilibrium
Energy Increase temperature
Increase pressure
change
moves
moves
move
(forward left
right left
right
reaction)
Ale) + 2 B(g) = X(g) + Zig) exothermic
endothermic
Ple) + Qig) = 2 Xig)
exothermic
A2(g) = X(g) + 2(g)
endothermic
2 Pig) = 2 C(s) + D(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
1) Complete the table to show what would happen to the position of the following gas phase equilibri...
Questions


Mathematics, 04.05.2021 06:00


Social Studies, 04.05.2021 06:00



Mathematics, 04.05.2021 06:00

English, 04.05.2021 06:00



Social Studies, 04.05.2021 06:00


Computers and Technology, 04.05.2021 06:00









