

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
13.How many grams of phosphorus (P4) are needed to completely consume 79.2 L of chlorine gas accordi...
Questions



Social Studies, 01.10.2019 11:30

Mathematics, 01.10.2019 11:30


English, 01.10.2019 11:30


Biology, 01.10.2019 11:30

Biology, 01.10.2019 11:30

Mathematics, 01.10.2019 11:30



English, 01.10.2019 11:30

History, 01.10.2019 11:30





Mathematics, 01.10.2019 11:30

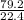 = 3.54mole
= 3.54mole 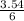 = 0.59mole of P₄
= 0.59mole of P₄ 

