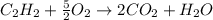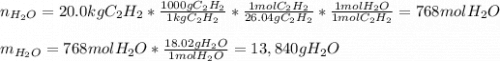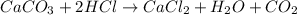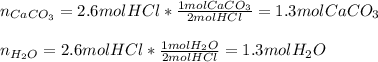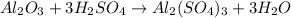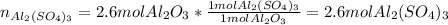Chemistry, 20.01.2021 14:00 matthewarroyo8988
Write the balanced equation and determine the information requested in each of the following.
1. The number of moles and the mass of water formed by the combustion of 20.0kg of acetylene, C2H2,in an excess of oxygen.
2. Limestone, caco3,dissolves in hydrochloric acid, Hcl, to form calcium chloride, carbon dioxide, and water. How many moles of Hcl are required to dissolve 2.6mol of caco3? How many moles of water are formed?
3.aluminiumoxide reacts with sulphuric acid to form aluminiumsulfate and water
A. how many moles of H2SO4 are required to react with 2.6mol of Al2O3
B. How many moles of Al2(So4)3 are formed?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
Write the balanced equation and determine the information requested in each of the following.
1. Th...
Questions



Computers and Technology, 24.11.2020 23:50



Mathematics, 24.11.2020 23:50


Mathematics, 24.11.2020 23:50

Mathematics, 24.11.2020 23:50


Mathematics, 24.11.2020 23:50


History, 24.11.2020 23:50



English, 24.11.2020 23:50

Health, 24.11.2020 23:50


Mathematics, 24.11.2020 23:50

Mathematics, 24.11.2020 23:50