
Chemistry, 20.01.2021 07:30 mannablofey23
Question 1: Which of the above electron dot diagrams is WRONG and why?
A) Fluorine F because it is supposed to have 8 Valence electrons
B) Carbon C because it supposed to have 8 valence electrons
C) Nitrogen N because it is supposed to have 6 valence electrons
D) Oxygen O because it is supposed to have 6 Valence electrons
Question 2: How many valence electrons does an atom of Argon have?
A) 8
B) 7
C) 6
D) 5
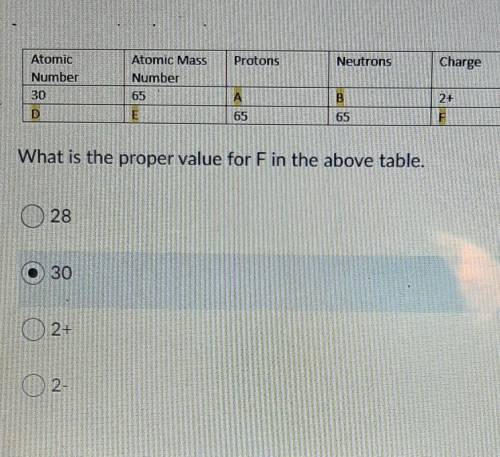
Question 3: What is the proper value for F in the above table. (Picture of table above..)
A) 28
B) 30
C) 2+
D) 2-
Question 4: What is the proper value for C in the above table. (Use the same picture as the previous question)
A) 28
B) 30
C) 32
D) 65

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 12:30
0.070g of hydride of carbon occupies 56cm^3 at s.t.p when vaporized and contained 14.29% by mass of hydrogen.what is the formula for the hydrocarbon
Answers: 1
You know the right answer?
Question 1: Which of the above electron dot diagrams is WRONG and why?
A) Fluorine F because it is...
Questions







Mathematics, 31.03.2020 02:26



Mathematics, 31.03.2020 02:26












