
Chemistry, 20.01.2021 04:40 alarimer3695
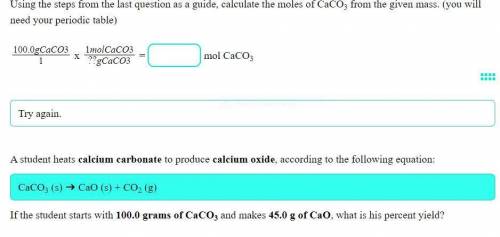
A student heats calcium carbonate to produce calcium oxide, according to the following equation:
CaCO3 (s) ➔ CaO (s) + CO2 (g)
If the student starts with 100.0 grams of CaCO3 and makes 45.0 g of CaO, what is his percent yield?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
A student heats calcium carbonate to produce calcium oxide, according to the following equation:
Ca...
Questions

Chemistry, 03.01.2020 01:31


Mathematics, 03.01.2020 01:31

Mathematics, 03.01.2020 01:31

History, 03.01.2020 01:31

Health, 03.01.2020 01:31


Mathematics, 03.01.2020 01:31




Mathematics, 03.01.2020 01:31








Social Studies, 03.01.2020 01:31



