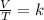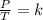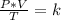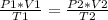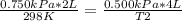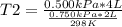Chemistry, 20.01.2021 01:50 KylaChanel4756
A sample of gas has a volume of 2.00 L and a pressure of 0.750 kPa when its
temperature is 25°C. If the volume is expanded to 4.00 L and the pressure reduced to
0.500 kPa, what must the temperature become?
379°C
397°C
379 K
397K

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
A sample of gas has a volume of 2.00 L and a pressure of 0.750 kPa when its
temperature is 25°C. If...
Questions

Health, 06.09.2021 14:00

Social Studies, 06.09.2021 14:00

Mathematics, 06.09.2021 14:00


Computers and Technology, 06.09.2021 14:00

Biology, 06.09.2021 14:00




Geography, 06.09.2021 14:00


Biology, 06.09.2021 14:00

Chemistry, 06.09.2021 14:00


Mathematics, 06.09.2021 14:00



Mathematics, 06.09.2021 14:00


Social Studies, 06.09.2021 14:00