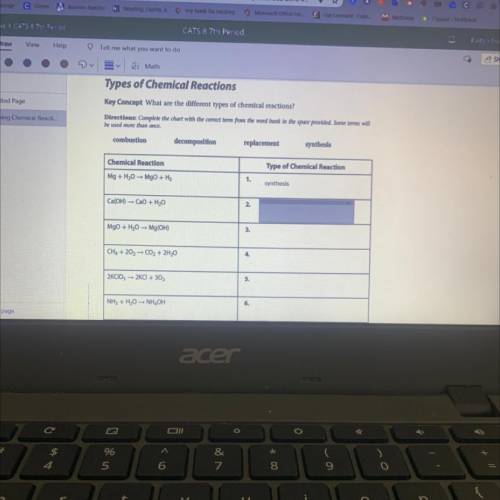
Ca(OH) =CaO + H2O
2.
MgO + H2O Mg(OH)
3.
CH4 + 202 - CO2 + 2H20
4.
2K...

Chemistry, 19.01.2021 21:10 Crtive5515
Ca(OH) =CaO + H2O
2.
MgO + H2O Mg(OH)
3.
CH4 + 202 - CO2 + 2H20
4.
2KCIO3 -- 2KCI+ 302
5.
NH3 + H20 NH OH
6.
Zn + 2HCI ZnCl2 + H2
7.
Add page
acer

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1
You know the right answer?
Questions


Mathematics, 21.02.2021 04:20

Biology, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20

History, 21.02.2021 04:20

Social Studies, 21.02.2021 04:20

Social Studies, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20



English, 21.02.2021 04:30

Physics, 21.02.2021 04:30

Arts, 21.02.2021 04:30

History, 21.02.2021 04:30

English, 21.02.2021 04:30



Computers and Technology, 21.02.2021 04:30

History, 21.02.2021 04:30

Mathematics, 21.02.2021 04:30



