Chemistry, 19.01.2021 19:10 Bangggggg6
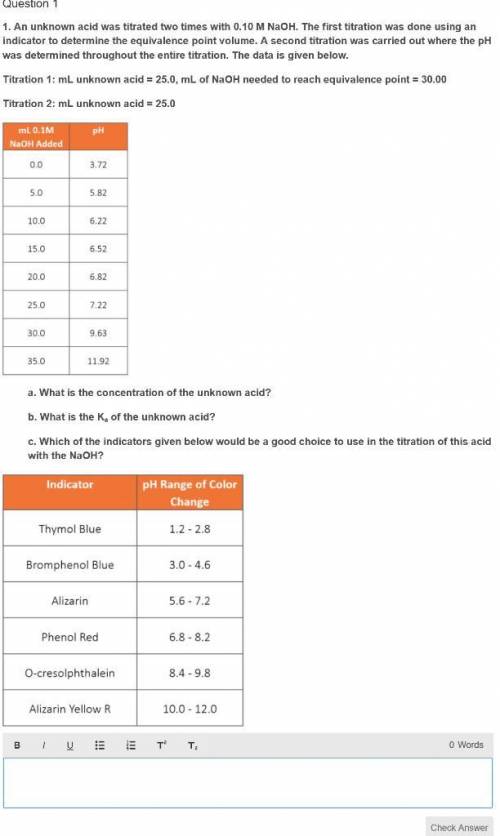
An unknown acid was titrated two times with 0.10 M NaOH. The first titration was done using an indicator to determine the equivalence point volume. A second titration was carried out where the pH was determined throughout the entire titration. The data is given below.
Titration 1: mL unknown acid = 25.0, mL of NaOH needed to reach equivalence point = 30.00
Titration 2: mL unknown acid = 25.0
mL 0.1M pH
NaOH Added
0.0 3.72
5.0 5.82
10.0 6.22
15.0 6.52
20.0 6.82
25.0 7.22
30.0 9.63
35.0 11.92
a. What is the concentration of the unknown acid?
b. What is the Ka of the unknown acid?
c. Which of the indicators given below would be a good choice to use in the titration of this acid with the NaOH?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
An unknown acid was titrated two times with 0.10 M NaOH. The first titration was done using an indic...
Questions





Mathematics, 14.01.2021 22:20


History, 14.01.2021 22:20



Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20




Mathematics, 14.01.2021 22:20


Chemistry, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20

Computers and Technology, 14.01.2021 22:20

Social Studies, 14.01.2021 22:20