

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
What is the [H ] of a solution of 0.001 M aqueous sulfuric acid (H2SO4) is H2SO4 ionizes in water ac...
Questions

Chemistry, 25.11.2021 07:10

Biology, 25.11.2021 07:10










Mathematics, 25.11.2021 07:10

Chemistry, 25.11.2021 07:10

Computers and Technology, 25.11.2021 07:10

Mathematics, 25.11.2021 07:10

Computers and Technology, 25.11.2021 07:10


Computers and Technology, 25.11.2021 07:10

Physics, 25.11.2021 07:10

World Languages, 25.11.2021 07:10

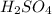 in water is:
in water is: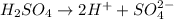
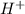 ions
ions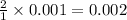 mole of
mole of 

