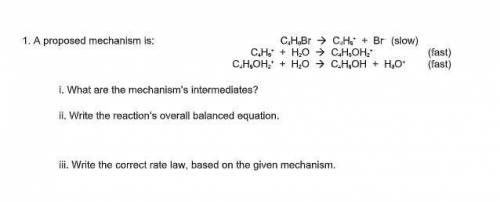
A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (f...

A proposed mechanism is:
C4H9Br --> C4H9^+ + Br^– (slow)
C 4H9^+ + H2O --> C4H9OH2^+ (fast)
C4H9OH2^+ + H2O --> C4H9OH + H3O^+ (fast)
i. What are the mechanism’s intermediates?
ii. Write the reaction’s overall balanced equation.
iii. Write the correct rate law, based on the given mechanism.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1


Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
Questions




Geography, 24.05.2021 22:30

Physics, 24.05.2021 22:30

Mathematics, 24.05.2021 22:30


Mathematics, 24.05.2021 22:30



Mathematics, 24.05.2021 22:30

Mathematics, 24.05.2021 22:30




Mathematics, 24.05.2021 22:30

Mathematics, 24.05.2021 22:30


Mathematics, 24.05.2021 22:30

Mathematics, 24.05.2021 22:30



