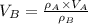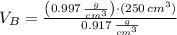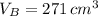Chemistry, 19.01.2021 14:00 Jazongamez1987
At 25.0 °C the density of liquid water is 0.997 g/cm3, but at -10.0 °C the density of solid water (ice) is 0.917 g/cm3. If a 250.0 mL sample of liquid water originally at 25.0 °C is frozen and cooled to -10.0 °C, what volume will the solid occupy?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
At 25.0 °C the density of liquid water is 0.997 g/cm3, but at -10.0 °C the density of solid water (i...
Questions






Mathematics, 03.07.2020 22:01



Mathematics, 03.07.2020 22:01








Health, 03.07.2020 22:01


Mathematics, 03.07.2020 22:01

History, 03.07.2020 22:01

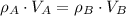 (1)
(1)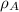 ,
, 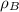 - Densities of water at 25 ºC and - 10 ºC, measured in grams per cubic centimeter.
- Densities of water at 25 ºC and - 10 ºC, measured in grams per cubic centimeter. 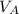 ,
, 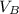 - Volume occupied by the water at 25 ºC and - 10 ºC, measured in cubic centimeters.
- Volume occupied by the water at 25 ºC and - 10 ºC, measured in cubic centimeters. 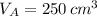 ,
, 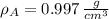 and
and 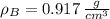 , then the volume occupied by the water at - 10 ºC is:
, then the volume occupied by the water at - 10 ºC is: