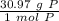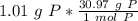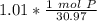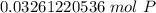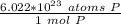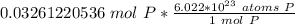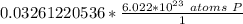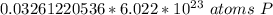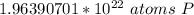Chemistry, 19.01.2021 06:00 TH3L0N3W0LF
How many atoms are present in a 1.01g sample of Phosphorus (P)? [Note: 1 mol = 6.022x10²³ atoms, molar mass of Phosphorus =30.97 g/mol) *
1 point
Captionless Image
3.48 x 10²¹ atoms
1.97 x 10²² atoms
1.04 x 10²⁶ atoms
3.36 x 10²⁴ atoms

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
How many atoms are present in a 1.01g sample of Phosphorus (P)? [Note: 1 mol = 6.022x10²³ atoms, mol...
Questions

Mathematics, 26.08.2020 19:01

History, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01




Geography, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01


Mathematics, 26.08.2020 19:01



Computers and Technology, 26.08.2020 19:01




English, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01


Mathematics, 26.08.2020 19:01